For Clinical Labs
In today’s diagnostic landscape, laboratories must deliver fast, reliable, and cost-effective results despite staffing shortages, tighter regulations, and rising technical complexity.

ue to the current coronavirus situation, there has been a lot of talk about COVID-19 tests. The general public is mostly concerned about the amount of testing, but there has also been a growing discussion around their reliability.
As quality of clinical tests is in the heart of our work, we wanted to share a few thoughts on the subject.
Here’s how you can use our Validation Manager to select diagnostics that actually works.
Before a new test can be taken to use, its performance must be evaluated. This is normally done by analyzing patient and control samples with known true values, and by statistically examining the quality of the results the test gives for these samples.
There are different ways to do that. A good starting point are the CLSI guidelines.
In Validation Manager, there are multiple studies that enable laboratories and test manufacturers to easily conduct their validations and verifications according to standards and guidelines.
Laboratories who do not yet use Validation Manager usually have a bunch of excel sheets for doing these calculations. While excel gives you quite much flexibility, it doesn’t really help you in your validations. You have to make sure by yourself, that all your data is really included in calculations. You cannot protect your data from mistakenly done manual edits. And later on it can be impossible to track, why your results are what they are. And after everything is done, you still have to go through all your data to manually construct your report. That’s a key reason why validations and verifications are often seen as tedious and wearisome.
With Validation Manager on the other hand, you have all your validations and verifications neatly in one place. Once you have planned what to do in your studies, all you need to do is import your data. And the best way to do that is day by day, always adding the most recent data on top of all your previous data. That way you won’t forget to include any results, and you can examine the report and make conclusions out of it as you go. What is convenient, is that you can import the results directly from instruments or laboratory information management systems. And once the results are imported, you even get your Word report with all the graphs and tables generated automatically.
With COVID-19, we are interested in whether the sample contains the virus or not. Therefore we are talking about qualitative tests, giving positive and negative results. (In Validation Manager we also have tools for verifying your quantitative tests, but this time we are skipping those.)
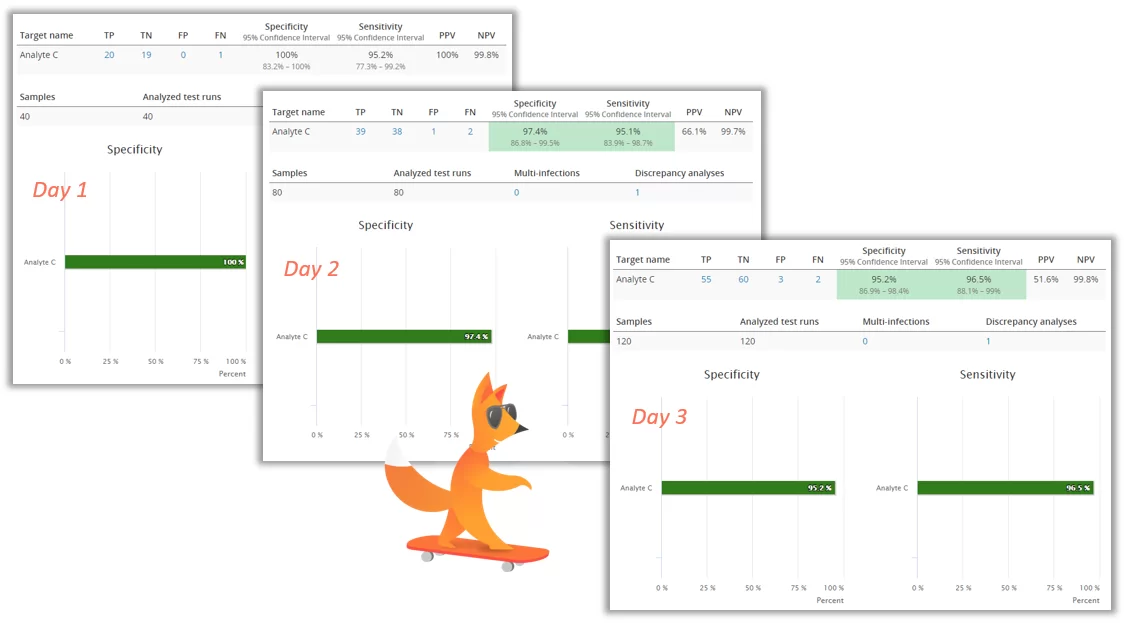
An essential tool for validating a qualitative test is to compare the results it gives to the results of a diagnostic accuracy criteria, i.e. the true values of the samples. In Validation Manager, this is done in Diagnostic Accuracy study. The key parameters that we are interested in are:
The beauty in using Validation Manager is that once you’ve planned your verification, all you need to do is upload your data and see how your report looks like. And you can add more data in your own pace.
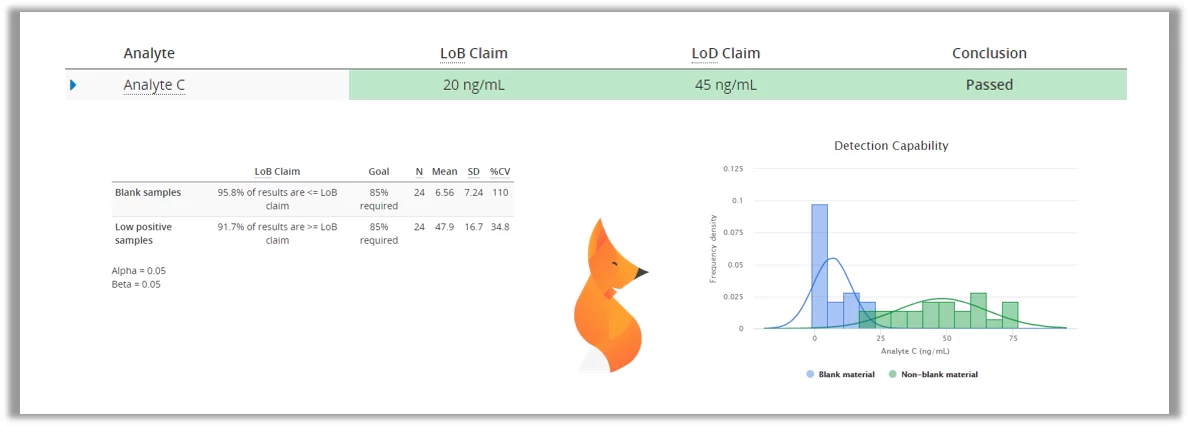
One thing we need to know about a test is how small amounts of the analyte it can reliably detect. In early stages of an illness, the amounts of viruses and especially their antigens are really small, so it is important to have an idea, how early a test is able to detect the condition.
With most laboratory tests, even negative samples have a high probability of giving low results that would indicate the presence of e.g. COVID-19 antigen. Therefore we need to measure a set of negative samples to establish limit of blank (LoB), a value under which the results should be interpreted as negative. After that we would measure a set of low positive samples to establish limit of detection (LoD), the lowest amount of antigen that can be reliably detected. In Validation Manager, these values can be easily calculated by importing your data to Classical Analytical Sensitivity study.
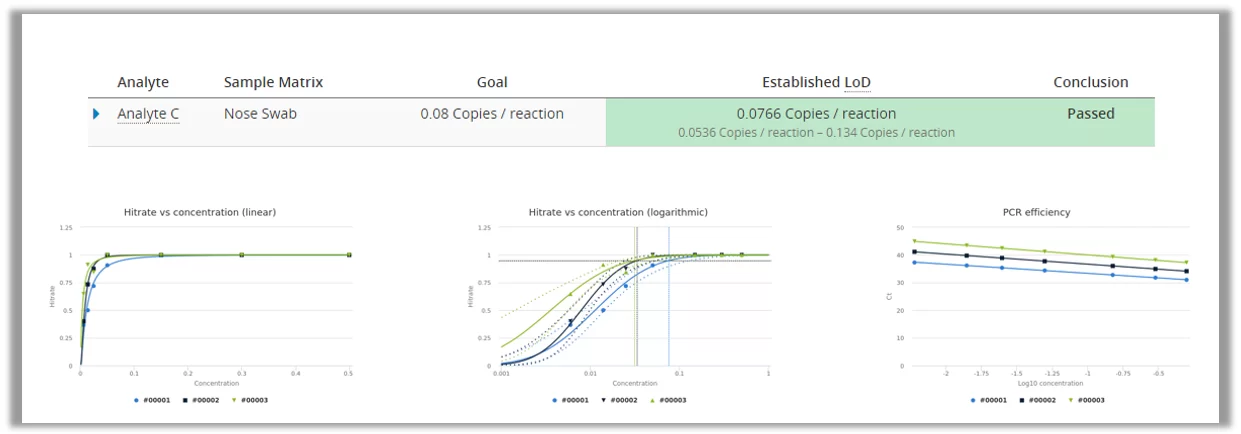
PCR technology is used for detecting the RNA of the COVID-19 virus from a sample. PCR is very good at detecting really small amounts of germs, and the technique of multiplying RNA/DNA makes sure that negative samples should not be mixed with low positive samples. Therefore we can assume that LoB=0 and only measure a dilution series of low positive samples to establish LoD using probit approach. This can be done easily by using Analytical Sensitivity study in Validation Manager.
To make things more complicated, we should also consider whether some other condition could cause the test to give positive results. (E.g. presence of another type of coronavirus. After all, before the current epidemic we already knew six species of human coronaviruses and a bunch that live in other animals.) To select the germs and medical conditions that could mistakenly be interpreted as COVID-19, you need knowledge of both the test and the conditions that could potentially be mixed with it. Then you need to collect samples with those conditions and test them with your new test. Analysing the results is not very difficult, as you only need to check that every sample gave a negative test result. But if you collect data through multiple days, managing the data and getting everything on one neat report might be a little painful. Uploading your data to Validation Manager as soon as you get it minimizes the time you would need to use organizing your data.
So whether you are developing or selecting COVID-19 diagnostics, Validation Manager can help you with effective validations and verifications. You will save time, and that may help you save lives.