For Clinical Labs
In today’s diagnostic landscape, laboratories must deliver fast, reliable, and cost-effective results despite staffing shortages, tighter regulations, and rising technical complexity.

We’ve recently published Quantitative Accuracy study. For the first time, we have enabled determining systematic error and random error in a single study and combining these values for estimating total error and measurement uncertainty. You can also use this study, for example, for tracking trends by importing your control results regularly into Validation Manager. On top of all this, we’ve made the smoothest workflow and the best looking report you’ve ever seen.
With Quantitative Accuracy study, one can estimate trueness, precision and accuracy of a method.
The samples used in the study should represent multiple sample levels, and these concentrations should be known.
To complete the study, CLSI EP10 protocol is recommended.
It is recommended to use both controls and patient samples. Controls are needed because their exact concentrations are known, unlike when comparing results from patient samples, where the reference results are affected by the uncertainty related to the reference method. On the other hand, control samples do not have the variation of the patient samples of your local population, and therefore patient samples are needed to describe the real laboratory use of the method.
If you’re not doing a validation but tracking test performance in routine QC, it’s naturally enough to use your control results.
In Qualitative Accuracy study, Validation Manager calculates following values:
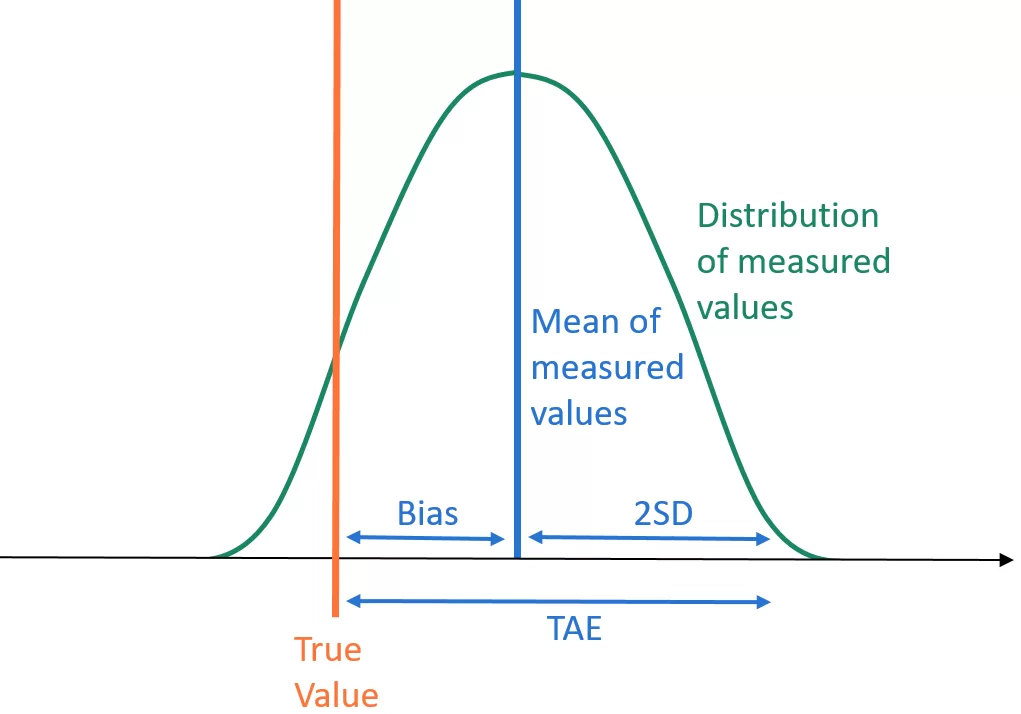
TAE is obtained by summing bias and two standard deviations
To give you a possibility to easily evaluate the calculated results, we’ve added an option to set goals for all these performance figures. Validation Manager compares calculated values to goals visualizing whether goals are met or not on each analyte-sample pair. So when you go through your results, it’s enough to look closely those results that do not meet the goals. As goals are analyte specific and each analyte is measured using several samples, this saves your time.
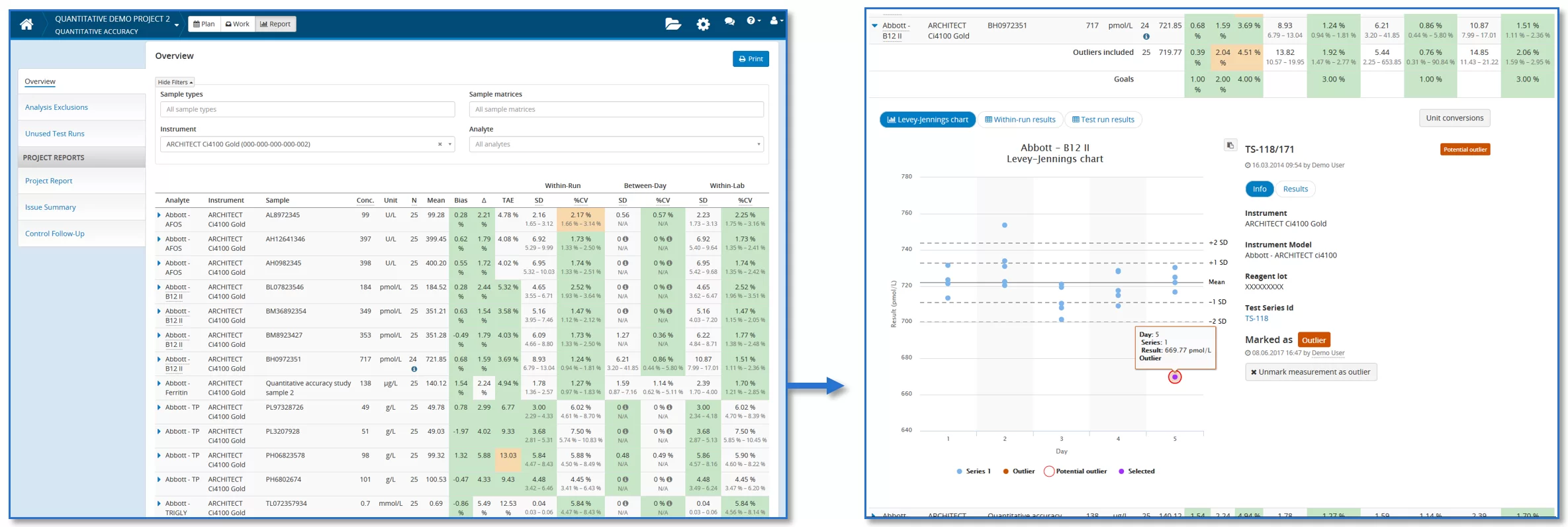
With Quantitative Accuracy study we don’t just give you a new tool for validations. We expand your possibilities as a user to get assistance from Validation Manager in periodic QC as well. We’ve also upgraded your user experience in quantitative studies. And with these new software design decisions, we have clear goals for further improvements that will be introduced in near future. So Quantitative Accuracy has brought us good new winds to head for an even smoother future of validations and verifications.